Abstract
Background: Survival rates of patients with APL have increased >90% with the introduction of all- trans retinoic acid (ATRA) and arsenic trioxide (ATO). However in addition to infections that are common during leukemia induction, bleeding and differentiation syndrome (DS) are frequently seen and are potentially fatal complications unique to APL. Renal failure is considered to be a manifestation of DS but can also occur secondary to infections. Herein, we report development of renal insufficiency (RI) during induction and outcomes in a cohort of patients managed by our program.
Methods: Between 1/2007 and 3/2017, we treated or co-managed a total of 210 patients.
Our new group (NG) consists of 138 patients which included, 120 patients treated on a prospective clinical trial in a network of Leukemia treatment centers established in Georgia, South Carolina and neighboring states between July 2013 and May 2016. This program was designed to decrease population wide induction deaths. The other 18 patients were referred to our institution from June 2016 to April 2017 and managed on the same algorithm as the prospective trial. Early Group (EG) consisted of 72 patients between 1/2007 and 5/2013 before initiation of this program.
We divided patients into four groups based on the CTCAE v.4.03 classification: Grade I - Creatinine (Cr) rise but less than 1.5 times baseline value; Grade II- Cr greater than 1.5 to 3 times baseline; Grade III- Cr greater than 3 times but less than 6 times baseline and Grade IV - Cr greater than 6 times baseline. Institutional review board approval was obtained and statistics are descriptive.
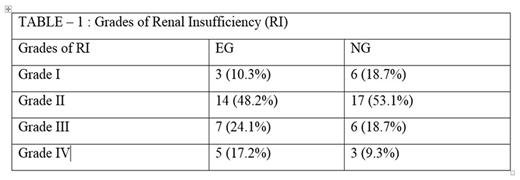
Results: All patients were treated at our institution in the EG while in the NG 62 (45%) were treated at our institution and 76 (55%) were managed in 33 outlying centers. The median age in EG and NG was 45 years (range 18-86) and 53 (range 19-84) and 61% and 54.3% were females respectively. RI of all grades developed in 29 (40%) in the EG and 32 (23 %) in NG. The breakdown in EG vs NG is given in Table1.
ATRA was the only therapy in 5 (17.2%) vs 5 (15.6%) patients in EG and NG. Other treatment for EG-RI included chemotherapy combined with ATRA in 19 (65.5%), where as in NG-RI only 6 (18.7%) received chemotherapy and ATRA. ATO was combined with ATRA in 4 (13.7%) of EG-RI and 19 (59.3%) of the NG-RI patients. Only 1 (3.4%) patient received ATRA, ATO and chemotherapy in EG-RI and 2 (6.2%) in NG-RI.
Complications among the patients with RI in EG and NG were: DS and infection in 9 (31%) vs 9 (28.1%), DS only in 4 (13.7%) vs 6 (18.7%); infection only in 12 (41.3%) vs 9 (28.1%) respectively. There were 4 (13.7%) vs 8 (25%) in EG and NG which had neither infection nor DS. Among those with DS, steroids were used in 100% of the patients in both EG and NG. There were 11 (37.9%) vs 6 (18.7%) early deaths in patients with renal insufficiency in EG and NG.
Conclusion: By our observation, RI is a frequent complication during induction in APL. Mortality in patients who develop RI tends to be high. Meticulous attention should be paid to kidney function during induction and the drugs should be appropriately dose reduced or held till resolution.
Arellano: Cephalon Oncology: Research Funding. Kota: Pfizer: Consultancy; Novartis: Consultancy; Xcenda: Consultancy; Incyte: Consultancy; Leukemia Lymphoma Society: Research Funding; Takeda Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.